More and more people these days are self described "science nerds" which is great, people should take an interest in science. Unfortunately, most people that describe themselves as science nerds are lacking in the basics and you need to know the basics when it comes to anything.
One of the most important things to know in most science fields is the periodic table of elements.
Having a deep knowledge of the periodic table is extremely beneficial especially in Chemistry.
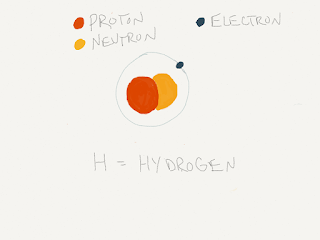
The modern periodic table was developed by Dimitri Mendeleev. In examining elements he found that they all had a different amount of Protons. The amount of Protons and the amount of Neutrons are the same for each. Also, the number of Protons is that same as the number of Electrons. Protons give the element mass and the electrons govern how elements will bond together. For example one Atom of Hydrogen has 1 of each. It conversely is the first element listed on the table and is also the lightest. Uranium is the last stable element on the list and is also the heaviest because it has the most Protons with 92.
So, now we know why the elements are numbered the way they are but why are they laid out in this strange looking table? Do the columns and rows have a meaning? They absolutely do. Starting with the first column with Hydrogen witch has 7 rows. Each row represents an additional electron shell or orbit. The amount of electrons will determine how many different orbits there will be ending with the last or outside orbit which will have between one and eight electrons orbiting with its own shell. That column ends with Francium which has 87 electrons divided up between 7th orbit with the 7th having only one electron.
Column 2 starts with Berylium at row two, which has four electrons. Since its on column two it has two electron on the outer shell and two orbits because its on row 2. If you have grasped that concept then I can tell you that the electrons on the outer orbit are called Valence Electrons. Valence Electrons are key to understanding on the element will interact with others.
Columns 3-12 are all the Transition Metals. These are the most common metals that you would encounter such as Zinc, Nickel, Iron, Copper and Gold. The amount of valance electrons does not follow the normal pattern. Since they are seemingly random one must relay on memorization for them. The electron shells however do correspond to which row they are in. For the most part the transition metals have 1-2 valence electrons.
Column 13 begins with Boron which now picks up where column 2 ended. Boron has 5 Electrons and its on row 2 so two orbits. The inner most orbit will always have two Electrons, so Boron has 3 Valence Electrons. This pattern will repeat itself for the rest of the table until the very last column, column 18, is reached which are the Noble Gases. Their outer Valence orbits are all full with 8 electrons each. The execption id Helium which only has two electron but since the first orbit can only hold two it is considered noble.
Noble Gases are sometimes called inert for example Argon because Argon cannot combine itself with anything neither can any Noble Gas. They need at least one space on the outer orbit and they simply don't have one.
Mendeleev was a very smart man. At the time he assembled the table there were many gaps and people made fun of him for it. He postulated correctly that there would be an element to fill the gap, it just hadn't been discovered yet and of course he was right.
If you need more of a reference I found a very good Periodic Table thats very interactive.
Dynamic Periodic Table


No comments:
Post a Comment